Supercapacitors are of high interest to engineers as an alternative energy storage technology to batteries because they are able to charge (and discharge) much faster.
In applications such as electric vehicles, rapid charging would be a big advantage to consumers who cite overnight replenishment of conventional batteries as a key discouragement to giving up fossil fuelled cars.
But the big weakness for supercapacitors is their inherently low energy density. For batteries, energy density is quantified as the amount of energy stored per kilogram. The best Lithium-ion battery can store about 720 kilojoules per kilogram (kJ/kg) whereas conventional supercapacitors struggle to exceed 100 kJ/kg.
Now researchers at University of California, Los Angeles (UCLA) claim to have overcome a limitation of capacitor electrodes that will improve the energy density of supercapacitors to a level approaching that of Lithium-ion batteries used in portable electronics and some electric cars.
"Our study demonstrates that our new graphene-based supercapacitors store as much charge as conventional batteries, but can be charged and discharged a hundred to a thousand times faster," Richard B. Kaner, Professor of Chemistry & Materials Science and Engineering at UCLA, said.
The electrodes of a capacitor form the "plates" which, when separated by a thin insulator, build up opposing charges in the presence of a voltage. Energy is stored in the electrostatic field between the charged plates.
The UCLA scientists’ development uses an expanded network of graphene - a one-atom thick layer of graphitic carbon – to replace the carbon electrodes of conventional supercapacitors.
The new electrodes exhibit high conductivity and provide larger and more "accessible" surface area than conventional capacitors. Boosting the surface area allows the supercapacitor to hold a greater charge and store more energy, increasing the energy density for a given component.
In a paper published in the journal Science, the researchers claim the new supercapacitors: "maintain excellent electrochemical attributes under high mechanical stress".
The open network structure of the graphene electrodes helps minimise the diffusion path of ions in the electrolyte used as the separator between the plates, which is said to be crucial for charging the supercapacitor.
In contrast, activated carbon forms very small pores that limit the diffusion of ions and compromise the ability to deliver ultra high power rapidly.
Because the graphene electrodes don’t require the binders or current collectors of conventional activated carbon supercapacitors – and can act as both the active material and current collector – the architecture of the devices is simplified, making them cheaper to make.
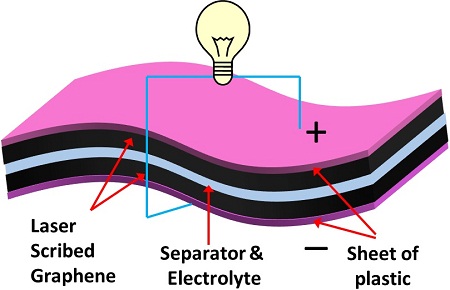
Commercially available capacitors comprise a separator sandwiched between two electrodes with liquid electrolyte that is either spirally wound and packaged into a cylindrical container or stacked into a button cell.
The UCLA research team replaced the liquid electrolyte with a polymer gel electrolyte that also acts as a separator, reducing device thickness and weight and further simplifying the fabrication process (see picture).




-160x160-state_article-rel-cat.png)
-160x160-state_article-rel-cat.png)





-160x160-state_article-rel-cat.png)
-160x160-state_article-rel-cat.png)




-160x160-state_article-rel-cat.png)



