To the lay person, surfactants and cleaning agents mean water polution, as they might not get retained at sewage plants, and if retained, the sludge that is spread on fields as fertilisers will poison the soil.
Solvents can be explosive, hard to handle and difficult to dispose of. Green Chemistry is the desired brainchild of a few Green activists, who do not want to recognise the dangers of reality in a world where the power of industry, and their influence on politicians, will ensure that Green Chemistry will never reach any market signifance, as it may disturb the big business of chemical conglomerates.
However, with all the negativity, public opinion is as wrong as it could be. In the last few decades, much progress has been made, Green Chemistry is a refreshing new technology which holds some positive economical factors. The much scolded chemical industry has jumped in, realising that they can improve their processes whilst simultaneously reducing their waste costs. In addition to saving financially, their image can be improved as well. It is the job of relevant industries, as well as politicians, to educate the public and their clientele accordingly.
So what does Green Industry mean?
Green Chemistry is defined as the type of chemistry that seeks to curb pollution, conserve energy and promote environmentally friendly production. At the same time risks to production and the product should be avoided.To achieve these objectives, the development and use of novel techniques is required.
On the other hand, environmental chemistry deals with the propagation, transformation and effects of chemical substances on the animate and inanimate environment. The Green Chemistry philosophy applies to all areas of chemistry, such as organic chemistry, inorganic chemistry, biochemistry, analytical chemistry and physical chemistry; it is therefore not confined to the industrial sector alone.
The concept of click chemistry is often referred to as a particularly green type of chemical synthesis. Click chemistry is to synthesise a target molecule faster and with smaller units as nature shows us.
Three important developments for Green Chemistry:
- the detection and use of supercritical carbon dioxide as a green solvent dissolved in water
- hydrogen peroxide for green oxidations and,
- the use of hydrogen for stereo-selective syntheses.
An example of the implementation of Green Chemistry is the oxidation reaction with supercritical water (supercritical water oxidation), and solvent-free reactions (Dry media reaction).
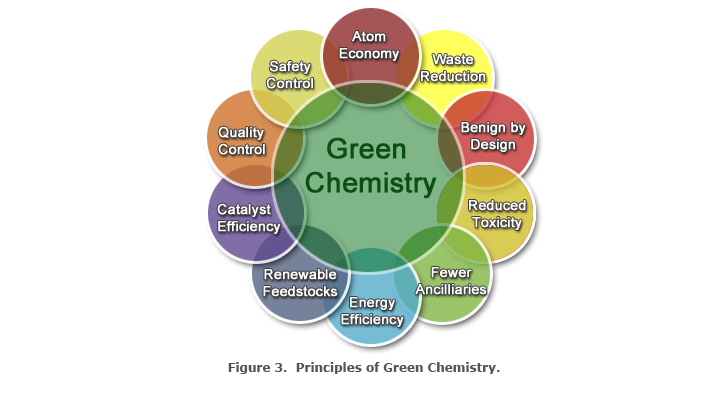
Another promising technology in the field of Green Chemistry is bioengineering. Numerous important chemicals can be prepared by synthesis in and with the help of microorganisms. In the future, eesearch literature on green chemistry will sooner or later come across the 12 principles of the Green Chemistry, which Paul Anastas of the Environmental Protection Agency and John C. Warner developed.
Even at the risk of repeating information, at minimum, they need to be cited here in an abbreviated form, for the benefit and understanding of those who are not intensely trained, but have basic knowledge of chemistry.
The 12 principles of Green Chemistry (or Green Chemistry Imperatives):
- avoid pollution: chemical syntheses, processes and reactors need to be designed to avoid dirt and contamination
- design safer chemical products: emphasise effective products that are less toxic than comparable materials
- produce less hazardous substances: create and use substances that pose no risk to humans and the environment
- use renewable raw materials as much as possible
- use of catalysts instead of stoichiometric reagents by minimisation of the reaction partners
- avoid unnecessary intermediate steps in chemical processes
- maximise the atom efficiency: design syntheses and reactions so that no, or only a few atoms or molecules of the initial reagents remain, or, so that no unwanted dangerous substances remain
- use safe solvents and safe reaction conditions: if possible, avoid the use of adjuvants
- increase energy efficiency: if possible manage reactions and processes at room temperature
- produce chemicals and side products: these can be degraded without harming the environment
- control all operations by real time management to prevent pollution and contamination, which will help to avoid waste
- minimise the risk of accidents
Conclusive and related definitions of Green Chemistry
Green Chemistry is more simply defined as the development and production of chemical products and the establishment of procedures that can replace or abandon the generation of dangerous products.
Green Chemistry is sustainable. Sustainable in the sense that it serves the needs of today’s generation without endangering the possibilities of future generations. The ecological term sustainability is always found in connection with Green Chemistry. It is also used in socio-economic and political contexts.
Green Chemistry Technologies
Having discussed all the theories surrounding the meaning of Green Chemistry, the associated technologies will be explored below, due to their affects on our daily lives.
Water purification
i.e. Replace chlorine disinfection by using Ascorbic acid
- laundry processes to reduce water usage
- using effective biodegradable detergents and surfactants
- cleaning processes in industry
- use of water based industrial cleaners
- biodegradable non-toxic surfactants
- antiviral and anti-bacterial products
- bandages, sutures, air filters, antiviral agents containing magnesium peroxide acetates
Examples non-organic solvents
Finally to round up Green Chemistry aspects, we will go a little deeper into chemical definitions:
Supercritical CO2 (scCO2)
One of the most important developments of the Green Chemistry is the development of the liquid and super critical CO2
Properties of scCO2:
- combination of properties from both the liquid and gas state
- at liquid-like densities, scCO2 exhibits low viscosity and high diffusion rates
high compressibility of the supercritical phase allows for solvent properties to be varied by small changes in temperature and pressure - scCO2 can be handled in standard high-pressure equipment on lab as well as in industrial scale
- scCO2 is non-toxic, non-flammable, and inexpensive.
- product isolation to total dryness is achieved by simple decompression
- scCO2 can be recovered and reused
Commercial applications of scCO2:
- natural product extraction like decaffeination
- polymer synthesis
- dry cleaning reducing the amount of organic solvents
Examples of scCO2 as Solvent in Synthetic Organic Chemistry:
- hydrogenation
- photochemical and radical reactions
- oxidations
- palladium mediated couplings
- biotransformation
- ionic liquids
Organic salts with melting points below 100°C, often below room temperature
Some applications of Ionic Liquids:
- for catalyst immobilization and recycling
- as electrolytes in electrochemistry Water
Water
Water, with all its assumed simplicity as a chemical reaction partner has become very important in Green Chemistry, with many realising that most of the world’s chemical reactions occur in aqueous media. Green Chemists have detected water again in their research and developments. Oxidations and hydrogenations are two examples of the revival of water as a reaction partner
Why Water?
- cost - water is the world’s cheapest solvent
- safety – it doesn’t get any safer than water
- some reactions just work better in water
Bio-Based Solvents and Surfactants
To close this article, Green Chemistry Ethyl lactate should be mentioned and closer described.
In recent years, the new classification “bio-based” can be added to industrial solvents. These green solvents are providing alternatives for conventional solvents when regulatory, environmental, or safety and health pressures are exerted.
They are based on agricultural or bio-based feedstock. As such, they not only provide environmental benefit from their use, but also avoid the more costly petroleum-based route to production. It is hoped that bio-based solvents and surfactants will avoid the high cost and significant price fluctuation associated with petroleum-derived solvents.
Several of the more common bio-based solvents that have recently been commercialised include ethyl lactate, d-limonene, and methyl soyate. All are produced from renewable resources such as corn, citrus, or soybeans.
Ethyl Lactate is a very common cleaning solvent is ethyl lactate. It is an environmentally benign solvent with properties superior to many conventional petroleum-based solvents. Unlike other solvents, which can damage the ozone layer or pollute groundwater, ethyl lactate is so benign that the U.S. Food and Drug Administration long ago approved its use in food products.
Ethyl lactate is produced by the reaction of lactic acid with ethanol. Both reactants are produced from agricultural materials. Lactic acid is produced by fermentation of lactose via specially developed bacteria, and ethanol is produced from corn.
Many bio-based surfactants and solvents are now developed continuously for more applications. All the major chemical houses are already offering wide varieties.
Smaller application oriented companies offer special formulations for their clients in various industries. As a result, the progress of Green Chemistry when it comes to solvents and surfactants can no longer be stopped.
Sources and links:
Mary Kirchhoff, Green Chemistry Institute
Principles of clean Chemistry Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998; pp 30.
Matthew J Scott Malcolm N Jones The biodegradation of surfactants in the environment
http://www.acs.org/content/acs/en/greenchemistry/what-is-green-chemistry/principles/green-chemistry-principle--5.html
Safer Solvents and Auxiliaries Dr. Concepcíon (Conchita) Jiménez-González, Director, Operational Sustainability, GlaxoSmithKlin
Oakes, R. S.; Clifford, A. A.; Rayner, C. M. “The Use of Supercritical Fluids in Synthetic Organic Chemistry” J. Chem. Soc., Perkin Trans. 1, 2001, 917–941.
Rideout, D. C.; Breslow, R. J. Am. Chem. Soc. 1980, 102, 7816.
Gajewski, J. J. In Organic Synthesis in Water; Grieco, P. A., Ed.; Blackie Academic & Professional: London, 1998.