On 31 March a long overdue change to the regulation of dietary supplements comes into force. It's a move that will bring the regulation of food-type dietary supplements – or supplemented foods – up to date with the myriad ways we can 'boost' our diets in the 21st century.
The change will reassure consumers that health and sports foods meet the same labelling and composition standards as other foods in the general food supply. The change will also mean that manufacturers of supplemented foods will be able to take advantage of technological advances in food manufacturing previously closed to them by the more restrictive rules of the old dietary supplements regime. Most importantly, the change provides clarity in an area where the distinction between 'food' and 'medicine' was becoming increasingly muddied.
Essentially, the new rules will distinguish 'food-type' from 'therapeutic-type' dietary supplements. The existing Dietary Supplement Regulations 1985 will continue to cover therapeutic-type dietary supplements pending updated therapeutic products legislation, and will move to be the responsibility of the Ministry of Health. Food-type dietary supplements will come under a new supplemented food standard – the New Zealand Food (Supplemented Food) Standard 2010 – which will be administered by the New Zealand Food Safety Authority (NZFSA).
In 1985, when the dietary supplement regulations were issued, few of us imagined that nutritional supplements would come in any form other than a pill, liquid or powder to be taken in addition to food. The regulations define dietary supplements as 'any amino acids, edible substances, foodstuffs, herbs, minerals, synthetic nutrients, and vitamins sold singly or in mixtures in controlled dosage forms as cachets, capsules, liquids, lozenges, pastilles, powders, or tablets, which are intended to supplement the intake of those substances normally derived from food'.
The regulators of the day didn’t foresee the plethora of highly-fortified cereal bars, fruit juices, yoghurts or protein drinks, selling on the market today as 'health' or 'sports' foods. Today, the dietary supplements industry as a whole is estimated to be worth almost half a billion dollars to the New Zealand economy. Our exports of dietary supplements are valued at more than $300 million.
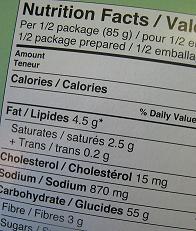
The new supplemented food standard has been designed to align as closely as possible with the Code, especially in terms of food labelling and composition. The dietary supplement regulations have fairly rudimentary requirements around labelling, mostly relating to active ingredients and dosage.
Under the new standard, consumers will have the added protections of mandatory allergen information, labels to be in English, nutrition information panels, and warnings labels for substances that have associated risks, such as caffeine. Any products containing caffeine that meet the definition of the Formulated Caffeine Beverages Standard, which is part of the Code, will continue to be regulated under that standard. Supplemented foods will not be allowed to contain intoxicating substances, such as alcohol or those used in party pills.
The ban on therapeutic claims that exists under the dietary supplement regulations will continue under the supplemented food standard. Any product that wishes to claim therapeutic (health) benefits has to provide proof, and is regulated under the Medicines Act. As a food regulator, NZFSA has neither the mandate not the capacity to regulate therapeutic products.
The change has been welcomed by manufacturers, many of whom are already voluntarily labelling supplemented foods to comply with the rules for food, which are set out in the joint Australia-New Zealand Food Standards Code. The changes allow them to use substances that are approved under the Code, such as a wider range of intense sweeteners, than is permitted under the dietary supplements regulations.
Those who are still manufacturing food-type products under the dietary supplements regulations now have two years to make the switch. This two-year transition period will keep compliance costs in check by allowing manufacturers to update product labels and formulations within normal product cycles.